Europe Ataxia Market Size, Growth, and Forecast to 2029
Introduction
The Europe Ataxia Market represents a crucial segment of the neurological disorder treatment industry, focusing on the diagnosis, management, and therapeutic development for ataxia—a group of disorders characterized by impaired coordination, balance, and speech. Ataxia arises from damage to the cerebellum, spinal cord, or peripheral nerves, leading to progressive motor dysfunction. The European market plays a significant role in global ataxia research, with advancements in genetic therapies, molecular diagnostics, and rehabilitation technologies reshaping patient outcomes.
Learn how the Europe Ataxia Market is evolving—insights, trends, and opportunities await. Download report: https://www.databridgemarketresearch.com/reports/europe-ataxia-market
The Evolution
Historically, the management of ataxia in Europe was limited to symptomatic treatment, focusing on physical therapy, speech rehabilitation, and assistive devices. In the late 20th century, the development of neuroimaging technologies like MRI and CT scans significantly improved diagnostic accuracy. The 2000s witnessed progress in identifying genetic mutations responsible for hereditary ataxias, such as Friedreich’s ataxia and spinocerebellar ataxias (SCAs).
Key innovations, including next-generation sequencing (NGS) and CRISPR-based gene editing, marked turning points in understanding the molecular basis of ataxia. European biopharmaceutical companies have been at the forefront of developing gene therapies aimed at addressing underlying genetic causes rather than just managing symptoms. Research collaborations among academic institutions, biotech firms, and national health systems have accelerated clinical trials and biomarker discovery.
The market has also seen a transition from traditional physical rehabilitation methods to digitally integrated rehabilitation platforms that combine virtual reality (VR), wearable sensors, and artificial intelligence (AI) to enhance motor learning and therapy outcomes.
Market Trends
The Europe Ataxia Market is undergoing substantial transformation driven by technological innovation, patient-centric care models, and increased funding for rare disease research. Several emerging trends define the market landscape:
1. Genetic Testing Expansion:
Comprehensive genetic panels and whole-exome sequencing are now widely used in clinical practice, enabling earlier and more accurate diagnoses of hereditary ataxias.
2. Growth of Gene and Cell Therapy Research:
Gene replacement and antisense oligonucleotide (ASO) therapies are under development for conditions such as Friedreich’s ataxia. European biotechnology firms are leading clinical trials targeting the FXN gene and other molecular pathways.
3. Digital Rehabilitation Tools:
Rehabilitation devices equipped with motion sensors and AI algorithms are improving patient monitoring, reducing hospital visits, and providing remote therapy options.
4. Public Awareness Campaigns:
Nonprofit organizations and patient advocacy groups across Europe are increasing disease awareness, facilitating patient support programs, and promoting early intervention strategies.
5. Integration of Personalized Medicine:
Precision medicine approaches are becoming central to treatment, with therapy protocols tailored to the genetic and clinical profile of each patient.
Challenges
Despite its progress, the Europe Ataxia Market faces multiple challenges that affect both patients and healthcare providers.
1. Limited Treatment Options:
Currently, no definitive cure exists for ataxia, and available therapies mainly address symptoms. This creates a significant unmet medical need.
2. High Research Costs:
The cost of developing genetic therapies and conducting clinical trials for rare diseases remains a major barrier. Pharmaceutical firms often struggle with limited patient populations and regulatory complexities.
3. Diagnostic Delays:
Many ataxia cases remain undiagnosed or misdiagnosed due to overlapping symptoms with other neurological conditions, leading to delayed treatment initiation.
4. Regulatory Hurdles:
European Medicines Agency (EMA) approval processes for orphan drugs are stringent, slowing down the introduction of innovative treatments.
5. Economic Disparities:
Differences in healthcare infrastructure across European countries affect access to advanced treatments, particularly in Eastern and Southern Europe.
Market Scope
The Europe Ataxia Market is segmented by type, treatment, diagnosis, and region.
By Type:
-
Friedreich’s Ataxia
-
Spinocerebellar Ataxia
-
Episodic Ataxia
-
Ataxia Telangiectasia
-
Acquired Ataxia
By Treatment:
-
Pharmacological Therapy
-
Gene Therapy
-
Physical and Occupational Therapy
-
Speech Therapy
-
Assistive Devices and Rehabilitation Technologies
By Diagnosis:
-
Genetic Testing
-
Imaging Techniques (MRI, CT Scan)
-
Blood and Metabolic Tests
-
Neurological Assessments
By End User:
-
Hospitals
-
Specialty Neurology Clinics
-
Diagnostic Centers
-
Research Institutes
Regional Analysis:
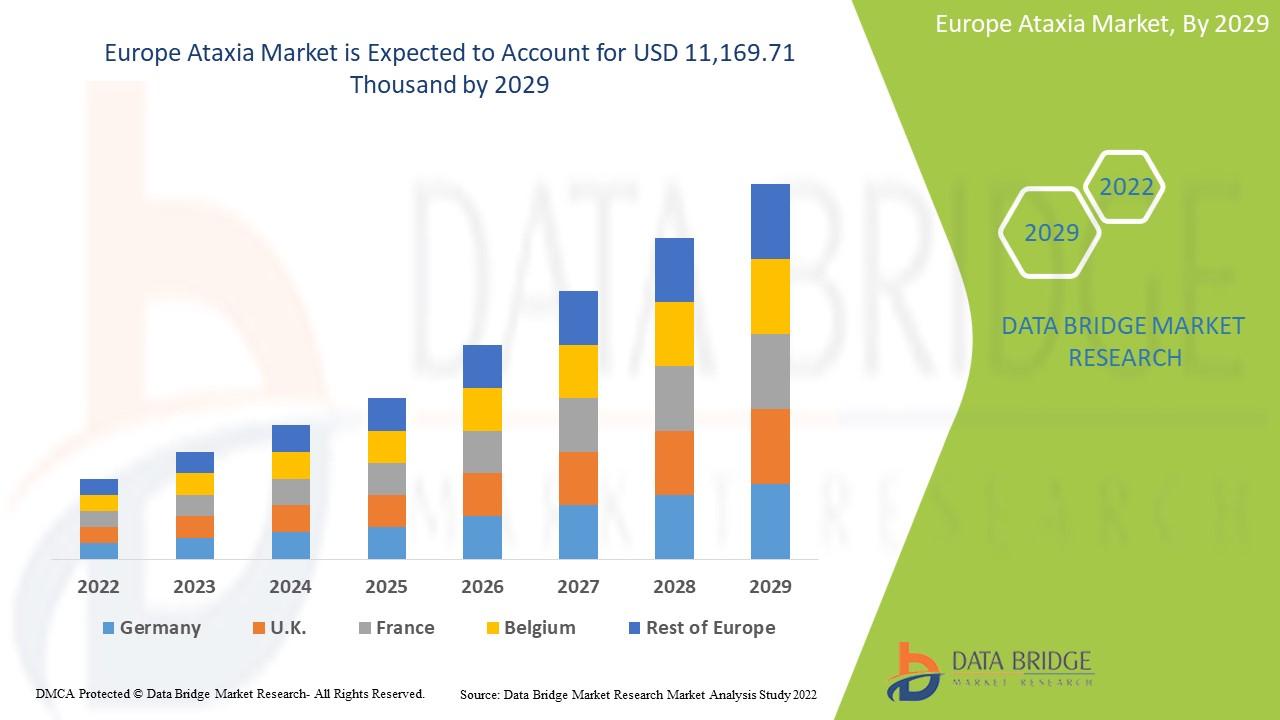
Western Europe: Countries like Germany, the UK, France, and Italy lead the market due to advanced healthcare systems and a strong focus on rare disease research. Germany holds a major share, supported by a network of specialized neurology centers and active patient registries.
Northern Europe: Scandinavian countries demonstrate high investment in biotechnology and digital rehabilitation tools, making them important growth contributors.
Southern and Eastern Europe: These regions are gradually improving healthcare access, supported by European Union (EU) funding and cross-border research collaborations.
Market Size and Factors Driving Growth
The Europe ataxia market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 5.8% in the forecast period of 2022 to 2029 and is expected to reach USD 11,169.71 thousand by 2029 from USD 7,424.22 thousand in 2021.
1. Rising Prevalence of Ataxia:
Increased awareness and improved diagnostic capabilities are identifying more cases of both hereditary and acquired ataxia.
2. Advancements in Genetic Research:
The identification of specific gene mutations and the success of gene-targeted therapy trials are major growth accelerators.
3. Expanding Healthcare Infrastructure:
Governments are strengthening healthcare networks and encouraging collaboration among European research centers to enhance rare disease management.
4. Supportive Policy Frameworks:
The European Commission’s initiatives for rare diseases and orphan drug designations have encouraged R&D investment in neurological disorders.
5. Growing Adoption of Digital Health Solutions:
The integration of telemedicine, wearable devices, and rehabilitation robotics supports continuous patient monitoring and home-based care.
6. Increased Funding and Partnerships:
Collaborations between pharmaceutical companies, academic institutions, and nonprofit organizations have resulted in accelerated drug discovery pipelines.
7. Aging Population:
Europe’s aging demographic is leading to a higher incidence of neurodegenerative conditions, contributing to the rising demand for ataxia-related healthcare services.
Opportunities in Emerging Regions
Eastern and Southern European countries present significant growth opportunities due to improving healthcare access and rising public investments in medical research. The establishment of new clinical research centers and patient registries in Poland, Czech Republic, and Portugal is enhancing the capacity to conduct large-scale clinical trials.
Telehealth platforms and digital rehabilitation tools are expected to expand across rural and low-resource settings, improving diagnosis and therapy access. Moreover, cross-border collaborations supported by the European Reference Network (ERN) are facilitating better resource sharing and harmonization of treatment guidelines.
Conclusion
The Europe Ataxia Market is entering a phase of accelerated innovation and patient-centric transformation. With advancements in gene therapy, molecular diagnostics, and digital rehabilitation, the industry is poised for sustained growth. While challenges such as high R&D costs and limited treatment options persist, supportive policy frameworks and public-private collaborations continue to drive research efforts.
The market’s future growth will depend on early diagnosis initiatives, increased access to precision therapies, and broader healthcare digitization. By 2035, the European region is expected to remain a global leader in ataxia research, offering innovative treatment pathways and improved quality of life for patients.
FAQs
1. What is the Europe Ataxia Market?
The Europe Ataxia Market focuses on the diagnosis, treatment, and management of ataxia—a neurological condition causing impaired coordination and motor control.
2. What is the market size of the Europe Ataxia Market?
The market was valued at approximately USD 320 million in 2024 and is projected to reach USD 640 million by 2035.
3. What are the main types of ataxia covered in the market?
The major types include Friedreich’s ataxia, spinocerebellar ataxia, episodic ataxia, and ataxia telangiectasia.
4. What are the key factors driving the market’s growth?
Advancements in genetic therapy, increased healthcare funding, and the expansion of digital rehabilitation solutions are the primary drivers.
5. Which countries are leading the Europe Ataxia Market?
Germany, the United Kingdom, France, and Italy lead due to strong healthcare infrastructure and advanced research capabilities.
6. What challenges does the market face?
High R&D costs, limited treatment availability, and complex regulatory approval processes are key challenges.
7. What is the forecast period for the Europe Ataxia Market?
The forecast period extends from 2024 to 2035.
8. What are the emerging opportunities in this market?
Emerging opportunities include gene therapy development, digital rehabilitation tools, and clinical research expansion in Eastern Europe.
Browse More Reports:
Global Cookie Mixes Market
Global Core Needle Biopsy Market
Global Corneal Tomography Market
Global Cosmetic Surgery and Services Market
Global Cosmetic Tubes Market
Global Counter Pulsation Devices Market
Global Cast Polypropylene (CPP) Packaging Films Market
Global Cranial Clamps Market
Global Cross-Linking Coating Agents Market
Global Customer Experience Management IoT Market
Global Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Market
Global Dairy Free Snacks Market
Global Data Management Advertising Software Market
Global Degenerative Disc Treatment Market
Global Dendritic Cell Therapy Vaccine Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"