Digital Transformation Spurs Growth in the Global eTMF Systems Market
Executive Summary Electronic Trial Master File (eTMF) Systems Market Opportunities by Size and Share
- The global electronic trial master file (eTMF) systems market size was valued at USD 1.84 billion in 2024 and is expected to reach USD 4.85 billion by 2032, at a CAGR of 12.90% during the forecast period
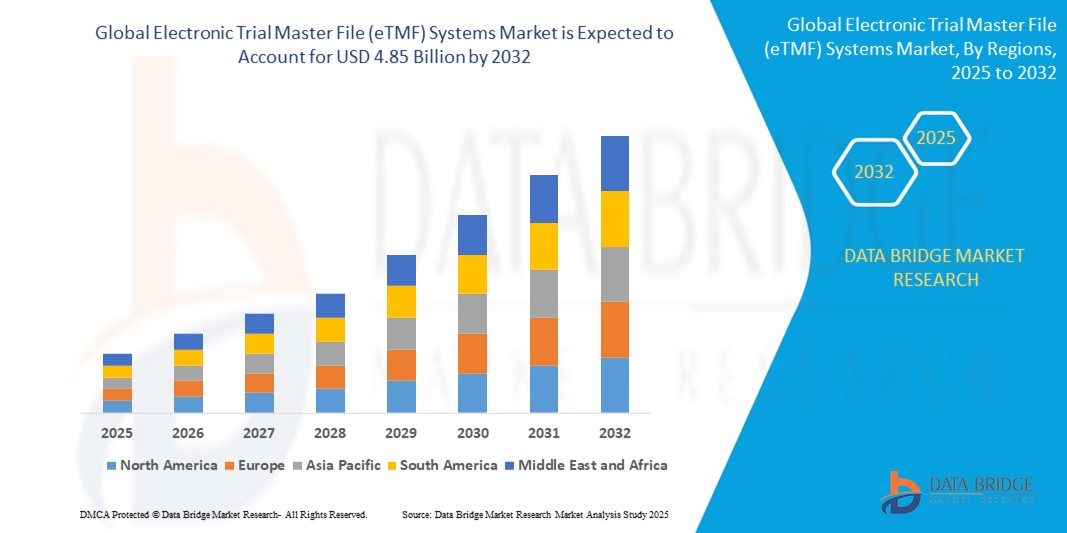
An international Electronic Trial Master File (eTMF) Systems Market report lends a hand to identify how the market is going to perform in the forecast years by providing information about market definition, classifications, applications, and engagements. A complete discussion about numerous market related topics in this market research report is sure to aid the client in studying the market on competitive landscape. This market report spans different segments of the market analysis that today’s business demand. The data and information collected with the research is generally quite a huge and is also in a complex form. However, such intricate market insights are turned into simpler version with the help of proven tools and techniques to provide it to the end users.
As per the DBMR team predictions cited in the Electronic Trial Master File (eTMF) Systems Market report, the market will grow with a specific CAGR value in the forecast period of 2023 to 2030. By taking into account strategic profiling of key players in the Electronic Trial Master File (eTMF) Systems Market industry, comprehensively analyzing their core competencies, and their strategies such as new product launches, expansions, agreements, joint ventures, partnerships, and acquisitions, the report helps businesses improve their strategies to sell goods and services. The credible Electronic Trial Master File (eTMF) Systems Market report contains market insights and analysis for Electronic Trial Master File (eTMF) Systems Market industry which are backed up by SWOT analysis.
Analyze top trends and market forces impacting the Electronic Trial Master File (eTMF) Systems Market. Full report ready for download:
https://www.databridgemarketresearch.com/reports/global-electronic-trial-master-file-etmf-systems-market
Current Scenario of the Electronic Trial Master File (eTMF) Systems Market
Segments
- Based on component, the eTMF systems market is segmented into services and software. The software segment is expected to dominate the market due to the increasing adoption of digital technologies in clinical trials, which require efficient software solutions for managing trial data effectively and securely.
- On the basis of deployment mode, the market is categorized into on-premise and cloud-based. The cloud-based segment is anticipated to witness rapid growth during the forecast period, as it offers benefits such as scalability, flexibility, and cost-effectiveness compared to traditional on-premise solutions.
- By end-user, the eTMF systems market is divided into pharmaceutical and biotechnology companies, contract research organizations (CROs), clinical research centers, and others. The pharmaceutical and biotechnology companies segment is expected to hold a significant market share, driven by increased investments in drug development and the need for efficient data management solutions.
Market Players
- Veeva Systems
- Oracle
- TransPerfect
- Aurea Software
- Phlexglobal
- DSG
- Montrium
- SureClinical
- MasterControl
- Wingspan Technology
These market players are at the forefront of driving innovation and technological advancements in the eTMF systems market. They are focusing on strategic partnerships, product launches, and acquisitions to strengthen their market presence and expand their customer base.
The electronic trial master file (eTMF) systems market is experiencing significant growth driven by the increasing complexity and volume of data generated during clinical trials. As the pharmaceutical and biotechnology industries continue to expand their research and development activities, the demand for efficient and secure data management solutions is escalating. The software segment within the eTMF systems market is poised to dominate due to the rising adoption of digital technologies in clinical trials. This trend necessitates sophisticated software solutions that can effectively organize and safeguard trial data, boosting the market for eTMF software providers.
Moreover, the deployment mode of eTMF systems is a crucial factor influencing market dynamics. The shift towards cloud-based solutions is gaining traction due to the scalability, flexibility, and cost-effectiveness they offer compared to traditional on-premise systems. Cloud-based eTMF systems enable seamless access to trial data from anywhere, facilitating collaboration among stakeholders and streamlining trial processes. As a result, the cloud-based segment is anticipated to witness rapid growth in the coming years, reflecting the broader trend towards cloud adoption across industries.
In terms of end-users, pharmaceutical and biotechnology companies represent a significant market segment for eTMF systems. These companies are ramping up investments in drug development, driving the need for efficient data management solutions to accelerate trial timelines and ensure regulatory compliance. Contract research organizations (CROs) and clinical research centers also play a vital role in the eTMF systems market, leveraging these solutions to enhance operational efficiency and deliver high-quality clinical trial services.
The competitive landscape of the eTMF systems market features several key players at the forefront of innovation and technological advancements. Companies such as Veeva Systems, Oracle, and TransPerfect are leading the market with robust software solutions tailored to the specific needs of clinical trial data management. Strategic initiatives such as partnerships, product launches, and acquisitions are common strategies employed by market players to strengthen their market presence and expand their customer base.
Overall, the eTMF systems market is poised for continued growth as the demand for streamlined and compliant data management solutions in clinical trials intensifies. With advancements in digital technologies and the increasing complexity of trial protocols, the role of eTMF systems in ensuring data integrity and regulatory compliance will only become more critical. Market players that can effectively address these evolving needs through innovative solutions and strategic partnerships will be well-positioned to capitalize on the expanding opportunities in the eTMF systems market.The electronic trial master file (eTMF) systems market is witnessing significant growth driven by the escalating demand for efficient and secure data management solutions in the pharmaceutical and biotechnology industries. With the increasing complexity and volume of data generated during clinical trials, there is a pressing need for sophisticated software solutions to organize and safeguard trial data effectively. This need is further amplified by the expanding research and development activities within these industries, pushing the market for eTMF software providers towards dominance.
The deployment mode of eTMF systems, particularly the shift towards cloud-based solutions, is a pivotal factor shaping market dynamics. Cloud-based solutions offer scalability, flexibility, and cost-effectiveness, making them increasingly preferred over traditional on-premise systems. The ability of cloud-based eTMF systems to provide seamless access to trial data from anywhere promotes collaboration among stakeholders and streamlines trial processes, driving rapid growth in this segment.
Pharmaceutical and biotechnology companies emerge as key end-users of eTMF systems, fueled by their heightened investments in drug development. The imperative to accelerate trial timelines and ensure regulatory compliance propels the adoption of efficient data management solutions within these sectors. Contract research organizations (CROs) and clinical research centers also contribute significantly to the eTMF systems market, leveraging these solutions to enhance operational efficiency and deliver high-quality clinical trial services.
The competitive landscape of the eTMF systems market is characterized by key players such as Veeva Systems, Oracle, and TransPerfect, who are driving innovation and technological advancements in the industry. These market leaders are strategically focused on forming partnerships, launching new products, and making strategic acquisitions to bolster their market presence and expand their customer base. By tailoring robust software solutions to the specific requirements of clinical trial data management, these companies are positioning themselves as industry frontrunners.
In conclusion, the eTMF systems market is set for continued growth as the importance of streamlined and compliant data management solutions in clinical trials continues to rise. With the evolution of digital technologies and the increasing intricacy of trial protocols, the role of eTMF systems in ensuring data integrity and regulatory adherence will become even more vital. Market players that can effectively address these evolving needs through innovation and strategic collaborations will be well-positioned to capitalize on the expanding opportunities within the eTMF systems market.
Access segment-wise market share of the company
https://www.databridgemarketresearch.com/reports/global-electronic-trial-master-file-etmf-systems-market/companies
Targeted Question Batches for Electronic Trial Master File (eTMF) Systems Market Exploration
- How is the Electronic Trial Master File (eTMF) Systems Market performing in current economic terms?
- What’s the outlook for market growth over the forecast window?
- How is the market structured by segment?
- Which brands have the largest footprint in the Electronic Trial Master File (eTMF) Systems Market ?
- What have been the most impactful recent product releases?
- Which regions and nations are assessed in the report?
- Where is the most dynamic market development occurring?
- Which country is predicted to lead the pack?
- What region holds a major stake in total revenue?
- What country has the most promising growth forecast?
Browse More Reports:
Middle East and Africa Malaria Treatment Market
Asia-Pacific Malaria Treatment Market
Europe Malaria Treatment Market
Asia-Pacific Recovered Carbon Black (rCB) Market
Middle East and Africa Medical Devices Market
Asia-Pacific Medical Devices Market
Asia-Pacific Airless Dispenser Market
Middle East and Africa Airless Dispenser Market
North America Airless Dispenser Market
Europe Microalgae Market
Middle East and Africa Microalgae Market
North America Microalgae Market
Middle East and Africa Digital Mining Market
Asia-Pacific Digital Mining Market
North America Digital Mining Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"

